News
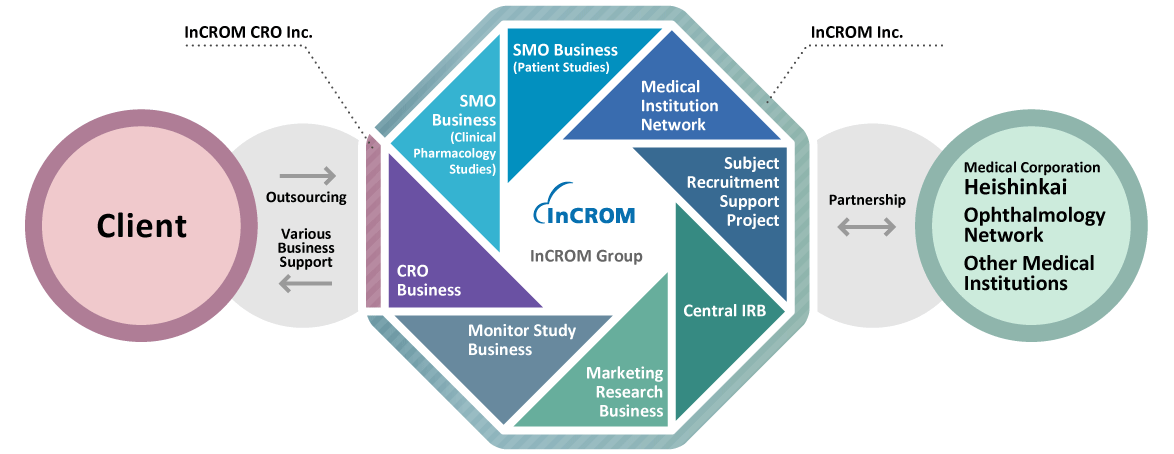
Pioneer in the Clinical Research Industry
As the pioneer established in 1983 (before the J-GCP enactment), InCROM has been providing SMO services to the pharmaceutical industry. To meet the ever-changing needs of our clients in the rapidly evolving industry, InCROM will continue to leverage on our decades of know-how and expertise to provide the highest quality of service.
With COVID-19: Challenging Times, Adaptive Solutions
COVID-19 has changed the world. Adapted to the new world, InCROM Group has implemented the following support services allowing the highest level of flexibility to clients during the pandemic.
If you have any questions or would like to schedule a meeting, please feel free to contact us.
If you have any questions or would like to schedule a meeting, please feel free to contact us.

Remote Meetings
Video Conferencing including all relevant in-house stakeholder experts allowing real-time effective discussion
Video conferencing is available for all of the eight business lines of InCROM Group. In addition to business development executives, other stakeholders such as:
• Investigators
• Clinical Trial Managers
• CRCs
• Subject Recruitment Staff
• Study Drug Managers
• CRO Staff
can also be present, allowing rea-time effective discussions no different from the conventional face-to-face meetings.
Examples of remote meeting systems that can be used: Zoom, Teams
• Investigators
• Clinical Trial Managers
• CRCs
• Subject Recruitment Staff
• Study Drug Managers
• CRO Staff
can also be present, allowing rea-time effective discussions no different from the conventional face-to-face meetings.
Examples of remote meeting systems that can be used: Zoom, Teams

Industry’s First Subject Panel & Cost Estimation System
InCROM Group owns the industry’s first subject panel search system that allows registered users to access to the system to search for subject information from the relevant subject panel(s). For unique or non-disclosed panel information, our subject recruitment specialists can be consulted upon request.
The system also houses a simple cost estimation function that can generate an estimate with the input of 10 items.
Click here to register.
The system also houses a simple cost estimation function that can generate an estimate with the input of 10 items.
Click here to register.

Remote Study Site Tour
Remote study site tour is another safe, user-friendly COVID-19 oriented option.
Remote touring of InCROM Group’s main affiliated study site, OPHAC Hospital, allows visitor to see the layout of each floor, equipment and other details conveniently.
Click here to schedule for a remote study site tour.
Click here to schedule for a remote study site tour.

Clinical Trials Information Subscriptions
Useful and regularly updated clinical trials information is readily available on the InCROM website.
In addition to the useful and regularly updated clinical trials information readily available on the InCROM website, registered users can also sign up for email subscriptions to have firsthand complimentary access to information such as –
・Live reports (e.g. COVID-19 trials, narcotic drug trials) from on-site investigators and clinical trial staff
・Clinical research relevant big data generated from the InCROM subject panels (e.g. distribution of HbA1c)
・Results from surveys (e.g. COVID-19 preventive measures) collected from registered users
・Case studies (e.g. Alzheimer’s Disease) derived from the InCROM subject panels
・Live reports (e.g. COVID-19 trials, narcotic drug trials) from on-site investigators and clinical trial staff
・Clinical research relevant big data generated from the InCROM subject panels (e.g. distribution of HbA1c)
・Results from surveys (e.g. COVID-19 preventive measures) collected from registered users
・Case studies (e.g. Alzheimer’s Disease) derived from the InCROM subject panels

Subject Recruitment Support Service
Subject recruitment optimization easily achieved via utilizing the InCROM-owned subject panels
Being the pioneer in clinical research, InCROM Group has been heavily and actively involved in the conduct of clinical trials for decades, leading to a strong record of subject recruitment. To date, the InCROM-owned subject panels have over 250,000 registrants mainly in the Kanto and Kansai regions and over 48,000 subjects were enrolled in the clinical trials supported. Not only does the registered user system include the necessary subject recruitment and registration functions, but it is also a robust healthcare resource platform containing ready-accessible information such as health foods, cosmetics, healthcare events, etc. in addition to subject recruitment and clinical trials information. The wide array of resources has been well received and enhances subject recruitment.
